New Method to Store Natural Gas Safely and Affordably
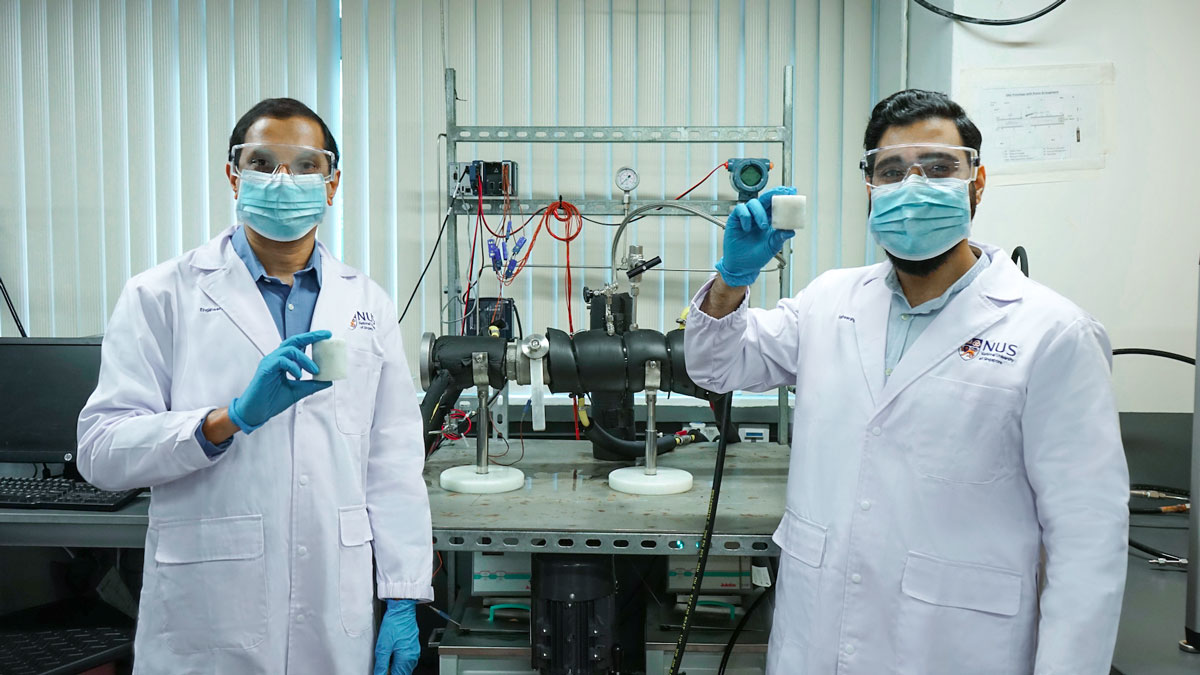
Natural gas is the cleanest of all fossil fuels but storing it safely and affordably remains a challenge. Now, engineers from the National University of Singapore (NUS) have devised a method to convert natural gas into a non-explosive solid that can be easily stored and transported. Using a novel, low-toxicity additive mixture they formulated, the conversion can be completed in just 15 minutes – the fastest time so far.
The NUS team was led by Associate Professor Praveen Linga from NUS Chemical and Biomolecular Engineering. Their paper was published in the journal Energy & Environmental Science on 27 October 2020.
Fast, safe, and portable way to store natural gas
The NUS team worked on a process of converting natural gas into a solid form known as gas hydrates, or combustible ice, which consists of molecules of natural gas trapped in ‘cages’ formed by water molecules. In fact, nature stores natural gas this way, but the process is extremely slow. Other researchers have previously managed to speed it up artificially, but they resorted to using highly toxic additives which are unsafe for both the environment and personnel involved.
The new additive mixture formulated by the NUS researchers contains L-tryptophan, well known as an essential amino acid in people’s diet. This muscle-building amino acid can also greatly speed up the caging of natural gas into solid hydrate. The NUS formulation produces the fastest reaction rate to date – more than twice as fast as existing standards – while being less toxic and safer to handle.
“Our breakthrough can really be put into perspective when you consider that it takes millions and millions of years for gas hydrates to form in nature, yet with our correct addition of secret ingredients to the system in small quantities, the same process can be effected in the laboratory in a matter of minutes,” Research Fellow Dr Gaurav Bhattacharjee, who worked on the project, said.
Moreover, the end-product is very stable and can be stored at -5 degree Celsius and atmospheric pressure, like the conditions of a home freezer. This is impressive considering that the natural gas is reduced in volume by close to 90 times. Alternative ways to store natural gas include liquefying it at about -160 degree Celsius or compressing it to almost 250 times atmospheric pressure. These approaches do not work at a large scale because either they are expensive or not so safe to store for long periods.
Scaling up for industrial use
The NUS researchers are now aiming to convert larger volumes of gas into smaller volumes of solid at a pilot-scale of 100 kilogrammes per day. If successful, this will enable the commercial adoption of the solidified natural gas technology and create a solid that is stable to store at atmospheric pressure. They hope to eventually scale it up for industrial use.
“This is especially relevant to natural gas importing countries like Singapore, where 95 per cent of electricity is generated using natural gas. The development of such gas storage technologies would help enhance the country’s energy security,” Assoc Prof Linga said.
This research was funded in part under the Energy Innovation Research Programme (ERIP), which is administrated by the Energy Market Authority (EMA) and funded by the National Research Foundation (NRF).
Notably, Assoc Prof Linga’s work was highlighted in the recently released Research Fronts 2020, a joint report by Clarivate and the Chinese Academy of Sciences to identify the top 100 research fronts for the period 2014 to 2019. According to the report, Assoc Prof Linga and his team have published the top three papers with the highest citations in the research front related to gas hydrates for “research progress on gas hydrate accumulation and mining technology”. These papers focused on the latest progress in creating natural gas hydrates, both in the laboratory environment and in mining field experiments, the limitations of the state-of-art natural gas exploration methods, and the challenges of large-scale exploration.
Source: National University of Singapore