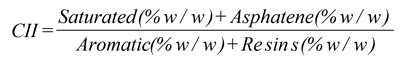Productivity-stimulation treatment for wells affected by stable emulsions
This paper presents a treatment solution for well stimulation where production is decreased due to the formation of stable emulsions. For this type of treatments, it is recommended to use an alkaline-surfactant solution for the treatment of asphalt-type oil emulsions with a high content of polar compounds. The presented solution (quaternary ammonium salt cationic surfactant in combination with sodium hydroxide) has the main objective to reduce the interfacial tension between water and oil and has a positive effect on oil recovery.
The studies on heavy, asphalt-type oils are specifically important for the petroleum industry first of all due to their composition. The problems related to emulsions forming and to the depositing of oil’s heavy components are present all along oil’s journey, starting from the reservoir to the processing installations.
Of particular importance are problems created by asphaltenes and resins, both from the point of view of laboratory investigations begin of the physico-chemical characterization, interfacial properties, polarity, to the investigation of the chemical structure using the structural models, as well as the exploitation of these crude oils, on wells.
The emulsions produced by wells are stabilized by emulsifiers (tension-active agents), which tend to agglomerate on the water-oil interfaces. The film formed by the tension-active agent on the interface decreases the interfacial tension between the two phases, which favours the dispersion of the discontinuous phase’s drops with the forming of emulsions. The natural emulsifiers in oil include heavy fractions as asphaltenes and resins, organic acids or organic bases. These components are considered as the main constituents agglomerating on the water-oil interface in the natural emulsions. Other emulsifiers may be present in the formation as a result of certain treatments applied to the well or coming from the filtrate of the drilling fluid. Also, the fine particles may act as emulsion stabilizers (mechanical stabilizers). They may include: fine particles of shale, asphaltenes, quartz, corrosion products and mineral scale.
The stability of the oil emulsions is also influenced by the very different dimensions of the water droplets; but the main influence of emulsion stability depends of the water percentage droplets of a certain size.
Considering this, there are three emulsion types: finely dispersed, very stable, with water drops around 20 µm; medium dispersed, with water drops of 20-50 µm and the last type, emulsions of big dispersion, unstable, with water drops of over 50 µm.
As an improved recovery process, the alkaline – tension-active solution system is recommended to oils with a high content of polar components (resins, asphaltenes), respectively organic acidity. The alkaline substances, in concentrations below 1%, dissociate integrally in water and lead to pH increases up to values of 9-13. The role of these inorganic bases is to react with the oleophilic acids of oil, transforming them to hydrophilic anions with active superficial properties. Thus, efficient tension-active substances, regarding their chemical structure and compatibility with oil, are formed in-situ. Here are some of the most frequently used alkaline substances in treating the wells with alkaline – tension-active solutions: sodium hydroxide, sodium ortho-silicate, sodium carbonate, soda, ammonium hydroxide.
This paper aims to develop a treatment solution for production stimulation of wells, where problems arise due to the formation of stable emulsions formed by the high content of asphaltenes from oil. Also, a specific test to verify the effect of this solution will be present on a physical model simulating that means to simulate the oil flow phenomenon from the productive formation to the production wells.
Experiments
Laboratory tests and analyses were performed on three samples of emulsified oil taken directly from the site from three production wells marked A, B and C. In this work, the following laboratory analyses and experiments were performed:
- Physico-chemical characterization of emulsified crude oil and crude oil;
- Selection of surfactants by performing crude oil emulsion burst tests and interfacial tension (IFT) analyses of oil/brine, respectively crude oil/surfactants;
- Testing the treatment solution on cores, under reservoir conditions (temperature and pressure) to assess the increase in the recovery factor of the crude oil.
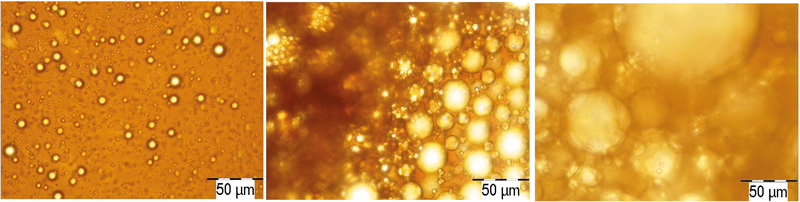
Analysis of emulsified crude oil – samples, taken from the wells
The characterization of the studied emulsions was done by micro-photos taken with a Cx41+Camedia C7070 microscope (Figure 1). To perform the next tests, the emulsions were cleaned by centrifugation using a 6-16 SIGMA heated centrifuge (650C and 3,200 rpm).
Three demulsifying agents were chosen to break the stability of emulsions A, B and C – anionic (AS), cationic (CS) and non-ionic (NS). The interfacial tensions (IFT) were determined for each of the three clean oils, along with the stability of emulsions by calculating the Emulsion Separation Index – ESI.
The ESI determination was done under the reservoir temperature of the wells the samples have been taken from (400C). The tension-active agents were tested in concentrations of 500 ppm. The work procedure consisted in adding the tension-active agent dose to 100 ml of emulsion sample, stirring and maintaining at work temperature for a certain time. The water volumes separated after 5, 10, 15 and 20 min are recorded, after which the samples are centrifuged for 20 min, under the same temperature.
Oil analysis – samples obtained after water separation
For characterizing the clean oils, complete oil analyses were performed (density, viscosity, distillation, chromatographic separation on SARA compounds – Saturated, Aromatic, Resins and Asphaltenes), including the organic acidity. Also, IFT determinations were performed for oil/water systems, respectively crude oil/surfactants systems.
The SARA analysis was carried out using the Iatroscan MK device. The organic acidity was determined by titration with alcoholic KOH standard solution, in the presence of Alkali Blue 6B, as an indicator. The ITF determinations were done using the Krüss tensiometer model DSA 100 and (with) the Pendant drop method, under atmospheric pressure and room temperature. The tension-active solutions were prepared using filtrated reservoir water, as 1% concentration of commercial product. Note that the laboratory tension-active samples are not 100% active substance, but each is provided by the manufacturer at a certain concentration of active substance. Based on the literature on the critical micellar concentrations for different tension-active substances and on the previous laboratory experiments, it was considered that the 1% concentration of commercial product is enough to evaluate the interfacial activity of the selected tension-active agents.
Displacement tests on synthetic cores with alkaline – tension-active solution
The displacement experiments with synthetic water and alkaline – tension-active agent solution on core-holding tubes were carried out using the thermal displacement installation of ICPT Campina. The tests performed for this work were done with sodium hydroxide, using this combination: 0.5% hydroxide + 1% tension-active agent.
The test laboratory conditions were as follows:
- The displacement experiments were done on physical models, using quartz sand with the grain size between 0.2 and 0.4 mm;
- The saturation fluids were oil and reservoir water from well C, and the alkaline – tension-active solution was prepared using reservoir water (1% NaOH + 1.5% CS);
- Tests were done at 400C and 10 bars.
The main stages were:
- Saturating of the porous space with reservoir water and setting the pores volume and the porosity;
- Water displacing with oil until reaching the irreducible water saturation, and setting the saturation state of the porous space;
- Injecting reservoir water, respectively alkaline – tension-active solution;
- Measuring the displaced oil volume and setting the saturation state of the porous space;
- Evaluating oil displacement efficiency by calculating the recovery factor.
There were performed three dynamic tests, as follows:
- Test 1: displacing oil with reservoir water;
- Test 2: displacing oil with alkaline – tension-active solution;
- Test 3: displacing oil with synthetic water, followed by alkaline – tension-active solution.
Results and discussion
a) The microscopic analysis of oil emulsions showed that their stability varies in this order A/B/C from highly stable to low stability. Figure 1 reveals that emulsion A has the finest dispersion of the water drops. Note that none of the emulsions separated free water.
Table 1
The water content and the size (distribution) of water drops in oil emulsions
| Emulsion sample | The water content [centrifugation] (% vol.) | Distribution of water drops in oil emulsions (μm) | Type of emulsion |
| Emulsion A | 48 | 20-30 | Very stable |
| Emulsion B | 52 | 30-60 | Medium stability |
| Emulsion C | 58 | 50-100 | Unstable |
The calculation of ESI was done using relation (1), and data are presented as a graph in Figure 2.
where ESI = emulsion separation index, w = water separation at a given demulsifier concentration/time as a percentage, and n = number of experiments.
b) The SARA analysis evaluated the asphaltenes behaviour in the crude oil by calculating the Colloidal Instability Index – CII, using formula (2). Literature estimates asphaltenes stability as follows: stable asphaltenes (CII<0.7), asphaltenes of moderate stability (0.7<CII<0.9) and unstable asphaltenes (CII>0.9).
Table 2 presents the results of SARA analyse. The analysed oils do not tend to deposit their asphaltenes, being thermodynamically stable. The problems given by the presence of asphaltenes are the emulsions stabilized by them and, in addition, the interfacial properties of oils are significantly altered by asphaltenes’ hetero-atoms.
c) Oil analyses were done on oil samples (impurities content 0.3 – 0.6 % vol.).
Table 2
Oil composition on compounds classes, according to SARA analyse
| Oil sample | Density (0API) | Saturated (%gr.) | Aromatic (%gr.) | Resins (%gr.) | Asphaltenes (%gr.) | CII |
| A | 12.11 | 35.23 | 31.89 | 25.26 | 7.61 | 0.75 |
| B | 14.86 | 38.20 | 31.75 | 22.61 | 7.43 | 0.84 |
| C | 14.72 | 34.50 | 30.60 | 21.22 | 10.11 | 0.86 |
The high viscosity of oils and the significant organic acidity (A – 1.54 KOH/g oil, B – 3.26 KOH/g oil and C – 5.78 KOH/g oil), recommends, for breaking the emulsions, the use of the alkaline – tension-active solutions. They are mainly recommended for the oils with a high content of polar components.
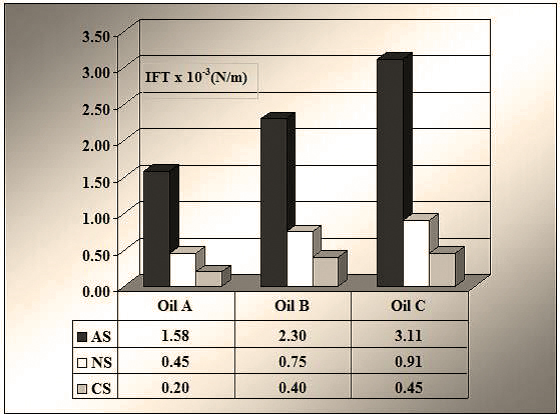
d) The IFT values between oil and reservoir water were the following: A – 28.54 mN/m, B – 22.64 mN/m and C – 17.20 mN/m.
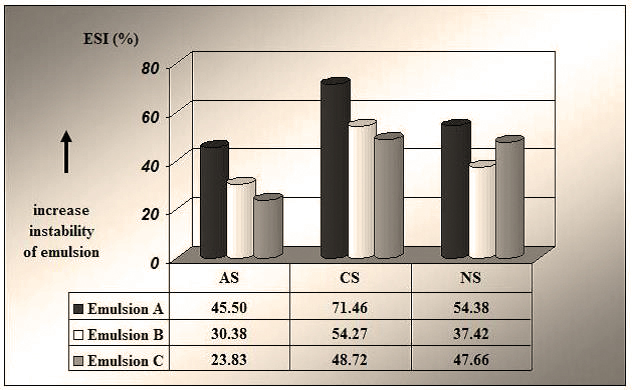
Figure 3 shows that the tested tension-active agents decrease the oil-water interfacial tension from an average value of 20-25 mN/m, to values between 1.5 and 3 mN/m for AS, respectively to values between 0.2 and 0.9 mN/m, for NS and CS. For the dynamic test on core, the CS tension-active agent was selected.
e) Models’ initial oil saturation varied between 75% and 82%, and at the end of tests oil saturation was 33% for the displacement using reservoir water followed by the alkaline – tension-active solution, as compares to 39% for the displacement only using reservoir water.
Table 3
Displacement tests results
| Test no. | Characteristics – physical model | Initial saturation (%) | Final saturation (%) | Recovery (%) | ||
| Diameter (cm) | Length (cm) | Porosity (%) | ||||
| Test 1 | 4.1 | 40 | 40.82 | SO=77.89 SW=22.11 | SO=39.30 | 49.55 |
| SW=60.70 | ||||||
| Test 2 | 41,54 | SO=82.07 SW=17.93 | SO=35.86 | 56.30 | ||
| SW=64.14 | ||||||
| Test 3
| 40.11 | SO=75.00 SW=25.00 | SO=38.57 SW=61.43 | 48.57 | ||
| SO=38.57 SW=61.43 | SO=32.86 SW=67.14 | 56.19 | ||||
If the porous space saturations are So (oil) and Sw (water), their relation is Sw = 1 – So, and the displacement efficiency using reservoir water, respectively alkaline – tension-active solution, is given by the recovery factor, expressed as the ratio between the oil volume collected during injection and the oil volume at the beginning of injection.
Recovery efficiency with reservoir water was between 48.6% and 50%. The displacement test using the alkaline – tension-active solution showed an approx. 6% increase of oil recovery.
Conclusions
The work investigated three heavy, asphalt-type oil samples, all with a high potential of forming stable emulsions in the production wells.
The following aspects were evidenced:
- Influence of size and distribution of water drops on oil emulsion stability;
- The cationic tension-active agents have a better influence on breaking oil asphalt-type emulsions than the anionic and non-ionic ones;
- For breaking the stable emulsions of the asphaltene-type oils and increasing wells’ productivity, recommend the alkaline – tension-active solutions.
Written with the help of Valentin Balteanu, OMV Petrom SA – ICPT Campina