ORNL Developed New Scalable Method to Improve Materials Joining in Solid-state Batteries
Scientists at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have developed a scalable, low-cost method to improve the joining of materials in solid-state batteries, resolving one of the big challenges in the commercial development of safe, long-lived energy storage systems.
Solid-state batteries incorporate a safer, fast-charging architecture featuring a solid-state electrolyte versus the liquid electrolytes in today’s lithium-ion batteries. A successful solid-state commercial battery system could provide at least two times the energy density of lithium-ion batteries in a much smaller footprint. The system would enable electric vehicles with vastly improved driving range, for instance.
One of the challenges in manufacturing solid-state batteries is the difficulty of getting materials to properly join and remain stable during repeated cycles of charging and discharging. Scientists studying methods in a lab to overcome this characteristic, called contact impedance, have so far focused on applying high pressures and other methods. But that process can lead to shorting and would need to be re-applied periodically to extend the battery’s life using an expensive aftermarket application.
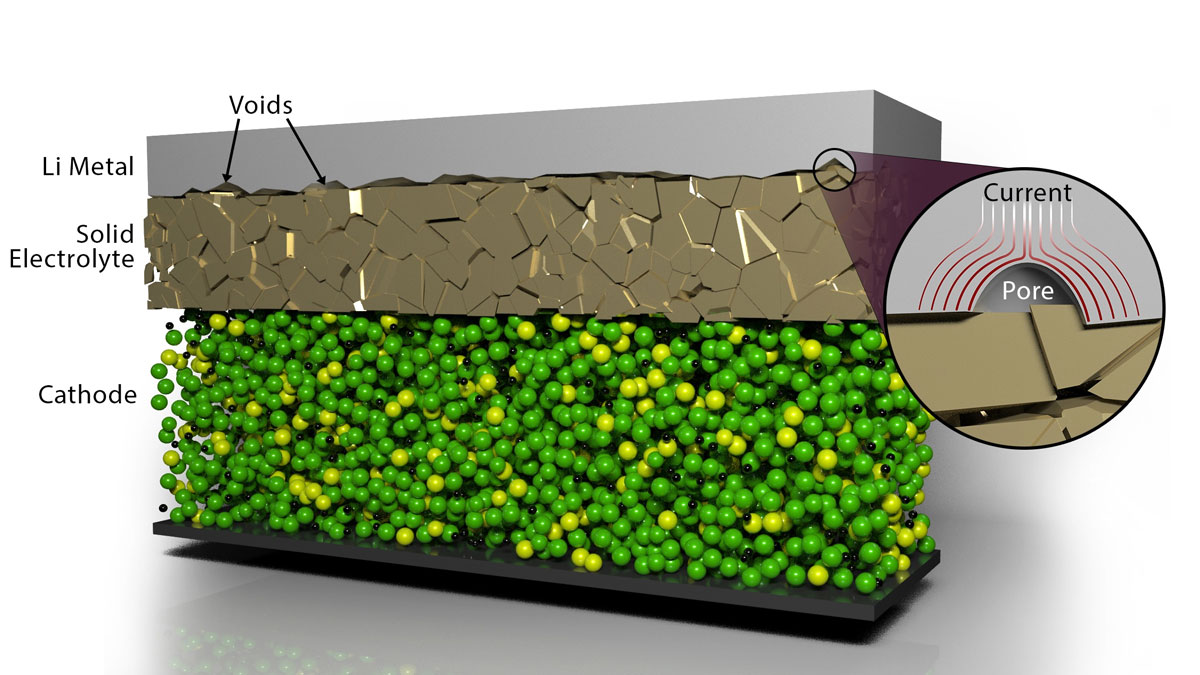
The electrochemical pulse the ORNL researchers used eliminates the voids that form when joining layers of lithium metal anode material with a solid electrolyte material: in this case the ceramic garnet-type electrolyte LALZO (Li6.25Al0.25La3Zr2O12). Applying short, high-voltage pulses led to increased contact at the interface of the materials while resulting in no detrimental effects, as detailed in ACS Energy Letters.
The non-destructive, low-cost pulsing method results in a local heat-generating current that surrounds the lithium metal-encased voids and causes them to dissipate. The team repeated experiments and advanced characterization of the materials, which revealed the battery components did not degrade after applying the pulsing method. This approach could be scaled to allow the solid-state battery to be removed and refreshed, bringing it back to nearly the original capacity.
“This method will enable an all-solid-state architecture without applying an extrinsic force that can damage the cell and is not practical to deploy during the battery’s usage,” said Ilias Belharouak, co-lead on the project and head of the Electrification Section at ORNL. “In the process we’ve developed, the battery can be manufactured as normal and then a pulse can be applied to rejuvenate and refresh the interface if the battery becomes fatigued.”
The idea for the method came from previous work in which ORNL battery researchers used electrochemical pulses to heal damaging dendrites that can form in solid electrolytes.
The research is ongoing, including experiments with more advanced electrolyte materials. ORNL’s multidisciplinary energy storage team is also working to scale up its breakthroughs to a working-scale solid-state battery system.
“Sometimes the things you see developed at the laboratory scale don’t end up working well together when you put them into cell architecture,” Belharouak said. “At ORNL, we try to build practicality into our work, using our deep bench of scientists and engineers to address the science gaps across scales for an approach that can be readily adopted by industry.”
Other scientists who worked on the project include co-lead Ruhul Amin, Marm Dixit, Rachid Essehli, Charl Jafta and David L. Wood, III of ORNL, and Anand Parejiya, a graduate student at the Bredesen Center for Interdisciplinary Education at the University of Tennessee, Knoxville.
The project used the capabilities of the DOE Battery Manufacturing Facility at ORNL. The research was funded by the DOE Office of Energy Efficiency and Renewable Energy’s Vehicle Technologies Office, as well as by ORNL’s Laboratory Directed Research and Development Program.