Phosphorus: A Critical Environmental Challenge
Phosphorus is essential for agriculture, food security and industry, but a critical challenge for water quality and the environment. The planet is faced with many sustainability issues. Fossil fuel sources become increasingly scarce, the increase in the concentration of greenhouse gases threatens the climate and drinking water becomes a resource more and more rare to find. Another problem, less known, but still very important, is the depletion of phosphorus.
Phosphorus extraction, processing and use involve substantial losses. This wastes a limited resource and contributes to severe environmental impacts such as eutrophication of freshwater ecosystems and development of dead zones in oceans. Sustainable phosphorus governance requires mapping stocks and flows to identify key points where we can minimize dissipation and increase recycling.
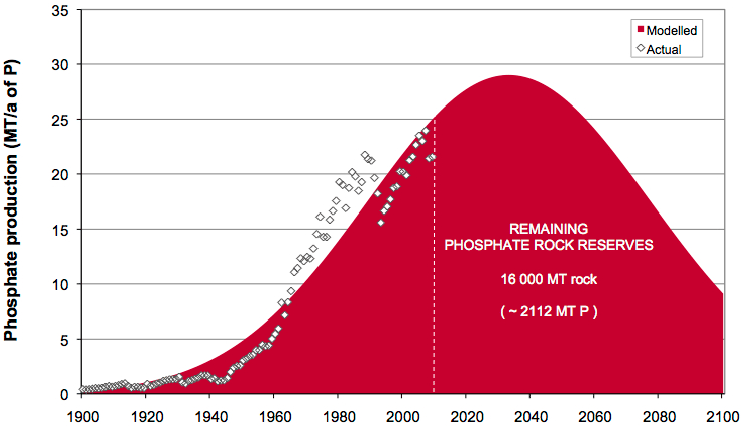
Phosphorus is not a renewable resource
In addition to agriculture, phosphorus has multiple uses in many sectors of the day-to-day life: pharmaceutical industry, personal care products, flame retardants, catalysts in the chemical industry, building materials, cleaning products, detergents, and food preservatives. Phosphorus is used in the production of special steels and bronzes, but also in the doping of semiconductors to increase their conductivity.
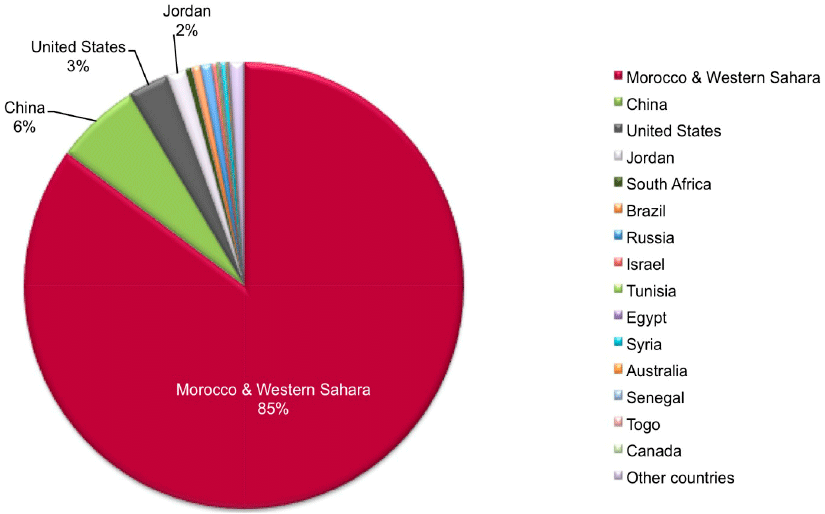
The phosphorus reserves are limited and not evenly distributed on the planet. The only large phosphate mines are located in Morocco, Russia, China and the USA. Various research groups have evaluated the lifespan of the global phosphate reserves and the results vary from 35 to 400 years. In the best-case scenario, the 400-year range is based on the discovery of new deposits.
As there is no even distribution of the mines and we have the insecurity of the capacity to meet demand, there is a major concern among the countries that do not have their own reserves, including all countries in the European Union. Also, as the independence of resources is one of the main strategic concerns for all governments, the depletion of phosphorus reserves could lead to geopolitical tensions.
An increase in the fertilizer price is an important threat to the developing countries: if farmers cannot afford fertilizers, it will result in a dramatic decline drop in crop yields and food production. Another problem related to the use of phosphorus is its damaging environmental impact. The excessive use of fertilizers leads to the leakage of phosphorus from agricultural fields into surface waters, which causes a phenomenon called eutrophication.
Aquatic death zones
Algae and toxins grow rapidly, lowering the level of light and oxygen in the water and leading to so-called ‘death zones’ in which fish and other aquatic organisms can no longer survive. The uncontrolled development of algae caused by high levels of phosphorus in the water has already created more than 400 ‘death zones’ near shores around the world.
Elimination of phosphorus from wastewater
Without phosphorus, life is not possible. However, human activities (agriculture, urban expansion, industries) deeply change its cycle. The consequences include the disruption of aquatic ecosystems, proliferation of algae, followed by their decomposition, consuming the oxygen needed by many species: this is called eutrophication. The eutrophication phenomenon is more or less important depending on the phosphate content in wastewater. Moreover, phosphorus is not a renewable resource. Globally, the future consumption estimates provide for the depletion of phosphate deposits within one to two centuries. Therefore, controlling the phosphorus flow in the environment is essential to restore degraded environments and ensure human nutrition.
How does the phosphorus cycle work?
Phosphorus is an essential component of living matter (DNA, cell membranes, enzymes, bone, ATP), but is a rare element in the wild (<0.1% of the mass of terrestrial rocks). It is found in the form of calcium, iron, and aluminum phosphates in volcanic and sedimentary rocks. On surfaces, phosphates are dissolved from the composition of rocks under the effect of the rainwater. The plants take the phosphates thus solubilized and use them to produce organic matter through the biosynthesis process. Phosphorus is then transferred along the food chain by plant consumption by animals. It is again solubilized by the decomposition of dead matter by microorganisms.
On a small scale (body of water, river, forest, pasture), phosphorus thus follows a succession of organic phases (living world) and minerals (after the decomposition of the living world). On a larger scale, phosphorus introduced into ecosystems by erosion is transported by rivers to the coastal areas they fertilize.
Phosphorus, which does not have a gas phase, has a high affinity for soil and sediment particles. Its natural cycle is not in balance between continental losses and accumulation in the ocean floor. This clearly differentiates it from the natural cycle of nitrogen, which takes place in a true loop between the atmosphere and the other compartments of the Earth. By introducing phosphates, man amplifies the imbalance of the natural phosphorus cycle.
Sources of phosphorus from human activities
Like all living things, humans need phosphorus. The growth of the world’s population has dramatically increased the need for food. In order to feed mankind, agriculture has seen a great growth in the last century and has gradually intensified and industrialized. In order to maintain a high productivity of agricultural systems in this context, phosphorus in the soil absorbed by crops must be artificially supplemented.
The use of phosphate fertilizers has increased widely in Europe and North America, with much higher inputs than the needs of cultivated plants. The use of polyphosphates in detergents has led to a dramatic increase in the amount of phosphorus in domestic wastewater. The recent massive introduction of phosphorus into the environment, the erosion of agricultural land and the increase in domestic wastewater are contributing to the rapid increase in phosphorus concentrations in aquatic environments. Significant stocks have been established in the environment, such as overfertilized agricultural soils or river sediments that accumulate phosphorus. Understanding this new distribution of phosphorus stocks on the planet’s surface is crucial for better management of this limited and non-renewable resource.
CICH Navodari, the only phosphate factory in Romania
Navodari Chemical Fertilizer Plant is a promoter of the latest fertilization technologies in Romania. Over the past few years, the company has certified and introduced in the market two technologies unique in Romania: nitrogen fertilizers with controlled release of the ammoniacal nitrogen part – NG range and phosphorus-based fertilizers or complexes with phosphorus enhancer – Amesal range, product ranges that are environmentally friendly.
In 2019, the plant in Navodari launched a new Nutri Top range, which incorporates three solutions developed by CICH – NGOOO, Amesal and Humic Extracts, bringing maximum efficiency to crops by providing humic acids, fulvic acids and amino acids.
Reduction of phosphorus input in aquatic environments
The environmental pollution caused by phosphorus, particularly in aquatic environments, has increased the interest in this element. It is considered to be the main culprit in the eutrophication process.
Etymologically, the word eutrophication means ‘well-nourished’. The term eutrophication refers to the consequence of hyperfertilization of water into nutrients (phosphorus and nitrogen), the ultimate point of which is ecological imbalance. Eutrophication manifests itself in an increase in algal biomass and deoxygenation of the water column, itself caused by heterotrophic mineralization of the organic matter produced.
Eutrophication affects rivers and lakes, as well as coastal areas. In addition, eutrophication can disrupt the structure of planktonic stands. For example, the proliferation of unwanted algae such as Dinophyceae and Cyanobacteria, some species of which can produce toxins. Deoxygenation can promote the release of pollutants associated with sediments (metals, micropollutants). Not to mention the economic impacts for swimming areas (toxic algae) and drinking water production (obstruction of pumping filters, the establishment of parasitic fauna in the networks, the development of tastes and smells incompatible with the concept of consumption etc.).
During the biosynthesis process, algae need carbon (C), nitrogen (N) and phosphorus (P). The growth of algae is conditional upon the ratio in which they take up nutrients (Redfield ratio C: N: P = 106: 16: 1). This ratio refers to the elementary molar composition of carbon, nitrogen, and phosphorus of an algae cell. If these elements are present in such proportions in the aquatic environment, there is no limit to growth. If one of these elements is missing, we are talking about a factor that limits the growth of algae (limitation by nitrogen or phosphorus, for example). In this ratio, phosphorus is often mentioned as the main factor limiting the growth of algae. Its responsibility is clearly proven in freshwater aquatic environments where nitrogen is largely in excess. Instead, for the coastal marine environments, nitrogen (in the form of nitrates) is often referred to as the main factor in eutrophication. This is the case, for example, in Brittany (France), where nitrates of agricultural origin are responsible for the spectacular proliferation of green algae on beaches.
Numerous cases of eutrophication have been reported worldwide since the 1960s. Initially, studies were conducted in lakes and reservoir dams, where the disturbances are strongest. The term oligotrophic refers to a nutrient-poor environment, with reference to its phosphorus concentration. A hypereutrophic environment is, on the contrary, the final stage of degradation. The term mesotrophic refers to a transient environment between ultra-oligotrophic and hypereutrophic. In rivers, the focus on eutrophication is more recent. Rivers are indeed considered to be self-purifying systems, capable of digesting and evacuating far downstream the disturbances suffered at a given point of the hydrographic network. However, the problem is very real in large rivers, with the proliferation of microalgae (phytoplankton), as well as in smaller ones, where aquatic plants proliferate (macrophytes). In addition, high nutrient inputs in rivers and streams inevitably have repercussions on coastal environments, lagoons, fjords, estuaries, which themselves are subject to serious global eutrophication problems.
Restoration of eutrophic environments
The first action in the fight against eutrophication was initially aimed at reducing phosphorus inputs. In a significant number of industrialized countries in Europe and North America, the collection of domestic wastewaters, the reduction of polyphosphates in washing liquids and the treatment of phosphorus in wastewater treatment plants have made it possible to significantly reduce the phosphorus inputs in aquatic environments.
The mechanisms of phosphorus transfer from agricultural sources are still being studied to identify the role of hydrology on transport modes. It is noted that the restoration process is very long (several decades) from diagnosis, decision making and action to visible results.
How far should we go to reduce phosphorus inputs in aquatic environments?
We will not radically change the way aquatic environments work because the issue of reducing phosphorus inputs has recently emerged in the European public debate. This is evidenced by the claims of professional fishermen, who have seen a decline in fish stocks for several years and require more phosphorus in the lake to increase the productivity of the ecosystem. While eutrophication is declining in Europe, the situation is critical in some emerging global regions, where extremely rapid urban growth and agricultural development still do not take environmental quality into account.
Increase in demand for phosphorus, a major challenge for humanity in the future
The social, economic, and environmental problems arising from the growing demand for phosphorus will be a major challenge for humanity in the future. A more sustainable and responsible use of phosphorus is crucial. About 80% of the mined phosphate rock is used to produce fertilizers, but most industries use much more than they need. The Food and Agriculture Organization of the United Nations (FAO) estimates that only 15 to 30% of phosphorus in fertilizers is actually taken up by plants, which is the largest loss in the entire circuit. The rest accumulates in the soil or reaches surface waters. For example, the amount of phosphorus currently accumulated in Dutch soil would be enough to supply the country with fertilizers for the next 40 years. Reducing nutrient waste in agriculture could be achieved through several technologies. Different types of crops can be selected taking into account soil type, climate and agricultural conditions to optimize phosphorus consumption.
In other industrial sectors, phosphates are reduced to elemental phosphorus, for example P4 (1 million tons per year). In this chemically pure form, phosphorus is much more reactive and can be easily converted to other families of phosphorus-based compounds. However, the processes for obtaining them are, in most cases, very expensive from an energy point of view.
The first step, the reduction of phosphate rock to elemental phosphorus, consumes 14 MWh per ton of phosphate, equivalent to the daily energy cost of a small nuclear reactor. The second step involves the transformation of elemental phosphorus into target compounds (through chemical oxidation processes that consume a lot of energy and produce waste). Thus, a direct transformation of the phosphate rock into target compounds would be incredibly advantageous in terms of energy and phosphorus consumption.
Phosphorus, a resource without substitutes
Phosphorus is widely used in agriculture and is an essential component of fertilizers and feed, but it is a resource without substitutes. Available reserves are limited, prices are volatile, and phosphorus is now largely wasted, which is a cause for concern about its future cost and availability, both in the EU and around the world. The question arises as to how we can ensure that reserves will continue to be available to future generations and how the adverse effects of phosphorus on the environment can be minimized. We are currently wasting this precious resource and create a pollutant. Through a more efficient use we will reduce its environmental impact and improve the security of supply. We can also create new business opportunities in the recycling sector.
At EU level there are consultations that aim to launch several debates on the use of phosphorus and how to increase the efficiency of using this resource. No specific legislation has been prepared on phosphorus, but European institutions and all stakeholders can express their views on this subject. Several options are suggested that could improve the current situation, for example the better targeted use of fertilizers and feed, reducing soil erosion and encouraging the recycling of phosphorus from manure, wastewater, and compost. The states are invited to explore the ways of encouraging the recovery of other phosphorus sources, such as food and biodegradable waste.
Faced with the serious challenges that future has in store for us, people rarely think about phosphorus. However, the guarantee that we will be able to feed the population of the planet is at least equally important as developing renewable energy and reducing greenhouse gases. To guarantee a long-term food security, it is vital that we change the way in which we currently use phosphorus.







